31 The last thing we should talk about

Capturing carbon dioxide from thin air is the last thing we should talk about.
When I say this, I am deliberately expressing a double meaning. First, the energy requirements for carbon capture from thin air are so enormous, it seems almost absurd to talk about it (and there’s the worry that raising the possibility of fixing climate change by this sort of geoengineering might promote inaction today). But second, I do think we should talk about it, contemplate how best to do it, and fund research into how to do it better, because capturing carbon from thin air may turn out to be our last line of defense, if climate change is as bad as the climate scientists say, and if humanity fails to take the cheaper and more sensible options that may still be available today.
Before we discuss capturing carbon from thin air, we need to understand the global carbon picture better.
Understanding CO2
When I first planned this book, my intention was to ignore climate change altogether. In some circles, “Is climate change happening?” was a controversial question. 1 As were “Is it caused by humans?” and “Does it matter?” And, dangling at the end of a chain of controversies, “What should we do about it?” I felt that sustainable energy was a compelling issue by itself, and it was best to avoid controversy. My argument was to be: “Never mind when fossil fuels are going to run out; never mind whether climate change is happening; burning fossil fuels is not sustainable anyway; let’s imagine living sustainably, and figure out how much sustainable energy is available.”
However, climate change has risen into public consciousness, and it raises all sorts of interesting back-of-envelope questions. So I decided to discuss it a little in the preface and in this closing chapter. Not a complete discussion, just a few interesting numbers.
Units
Carbon pollution charges are usually measured in dollars or euros per ton of CO2 so I’ll use the ton of CO2 as the main unit when talking about percapita carbon pollution, and the ton of CO2 per year to measure rates of pollution. (The average European’s greenhouse emissions are equivalent to 11 tons per year of CO2; or 30 kg per day of CO2.) But when talking about carbon in fossil fuels, vegetation, soil, and water, I’ll talk about tons of carbon. One ton of CO2 contains 12/44 tons of carbon, a bit more than a quarter of a ton. On a planetary scale, I’ll talk about gigatons of carbon (Gt C). A gigaton of carbon is a billion tons. Gigatons are hard to imagine, but if you want to bring it down to a human scale, imagine burning one ton of coal (which is what you might use to heat a house over a year). Now imagine everyone on the planet burning one ton of coal per year: that’s 6 Gt C per year, because the planet has 6 billion people.
Where is the carbon?
Where is all the carbon? We need to know how much is in the oceans, in the ground, and in vegetation, compared to the atmosphere, if we want to understand the consequences of CO2 emissions. 2
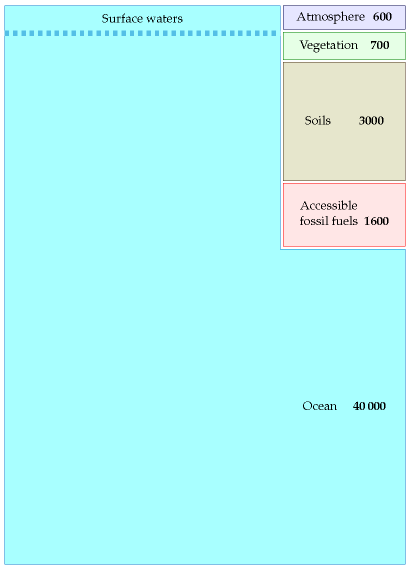
Figure 31.2 shows where the carbon is. Most of it – 40 000 Gt – is in the ocean (in the form of dissolved CO2 gas, carbonates, living plant and animal life, and decaying materials). Soils and vegetation together contain about 3700 Gt. Accessible fossil fuels – mainly coal – contain about 1600 Gt. Finally, the atmosphere contains about 600 Gt of carbon.
Until recently, all these pools of carbon were roughly in balance: all flows of carbon out of a pool (say, soils, vegetation, or atmosphere) were balanced by equal flows into that pool. The flows into and out of the fossil fuel pool were both negligible. Then humans started burning fossil fuels. This added two extra unbalanced flows, as shown in figure 31.3.
The rate of fossil fuel burning was roughly 1 Gt C/y in 1920, 2 Gt C/y in 1955, and 8.4 Gt C in 2006. (These figures include a small contribution from cement production, which releases CO2 from limestone.) 3
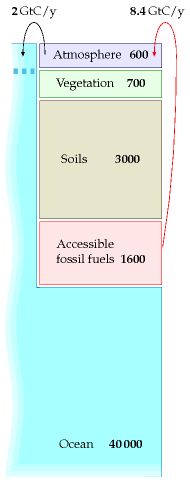
How has this significant extra flow of carbon modified the picture shown in figure 31.2? Well, it’s not exactly known. Figure 31.3 shows the key things that are known. Much of the extra 8.4 Gt C per year that we’re putting into the atmosphere stays in the atmosphere, raising the atmospheric concentration of carbon-dioxide. The atmosphere equilibrates fairly rapidly with the surface waters of the oceans (this equilibration takes only five or ten years), and there is a net flow of CO2 from the atmosphere into the surface waters of the oceans, amounting to 2 Gt C per year. (Recent research indicates this rate of carbon-uptake by the oceans may be reducing, however.) 4 This unbalanced flow into the surface waters causes ocean acidification, which is bad news for coral. Some extra carbon is moving into vegetation and soil too, perhaps about 1.5 Gt C per year, but these flows are less well measured. Because roughly half of the carbon emissions are staying in the atmosphere, 5 continued carbon pollution at a rate of 8.4 Gt C per year will continue to increase CO2 levels in the atmosphere, and in the surface waters.
What is the long-term destination of the extra CO2? Well, since the amount in fossil fuels is so much smaller than the total in the oceans, “in the long term” the extra carbon will make its way into the ocean, and the amounts of carbon in the atmosphere, vegetation, and soil will return to normal. However, “the long term” means thousands of years. Equilibration between atmosphere and the surface waters is rapid, as I said, but figures 31.2 and 31.3 show a dashed line separating the surface waters of the ocean from the rest of the ocean. On a time-scale of 50 years, this boundary is virtually a solid wall. Radioactive carbon dispersed across the globe by the atomic bomb tests of the 1960s and 70s has penetrated the oceans to a depth of only about 400 m. 6 In contrast the average depth of the oceans is about 4000 m.
The oceans circulate slowly: a chunk of deep-ocean water takes about 1000 years to roll up to the surface and down again. The circulation of the deep waters is driven by a combination of temperature gradients and salinity gradients, so it’s called the thermohaline circulation (in contrast to the circulations of the surface waters, which are wind-driven).
This slow turn-over of the oceans has a crucial consequence: we have enough fossil fuels to seriously influence the climate over the next 1000 years.
Where is the carbon going
Figure 31.3 is a gross simplification. For example, humans are causing additional flows not shown on this diagram: the burning of peat and forests in Borneo in 1997 alone released about 0.7 Gt C. Accidentally-started fires in coal seams release about 0.25 Gt C per year.
Nevertheless, this cartoon helps us understand roughly what will happen in the short term and the medium term under various policies. First, if carbon pollution follows a “business as usual” trajectory, burning another 500 Gt of carbon over the next 50 years, we can expect the carbon to continue to trickle gradually into the surface waters of the ocean at a rate of 2 Gt C per year. By 2055, at least 100 Gt of the 500 would have gone into the surface waters, and CO2 concentrations in the atmosphere would be roughly double their pre-industrial levels.
If fossil-fuel burning were reduced to zero in the 2050s, the 2 Gt flow from atmosphere to ocean would also reduce significantly. (I used to imagine that this flow into the ocean would persist for decades, but that would be true only if the surface waters were out of equilibrium with the atmosphere; but, as I mentioned earlier, the surface waters and the atmosphere reach equilibrium within just a few years.) Much of the 500 Gt we put into the atmosphere would only gradually drift into the oceans over the next few thousand years, as the surface waters roll down and are replaced by new water from the deep.
Thus our perturbation of the carbon concentration might eventually be righted, but only after thousands of years. And that’s assuming that this large perturbation of the atmosphere doesn’t drastically alter the ecosystem. It’s conceivable, for example, that the acidification of the surface waters of the ocean might cause a sufficient extinction of ocean plant-life that a new vicious cycle kicks in: acidification means extinguished plant-life, means plant-life absorbs less CO2 from the ocean, means oceans become even more acidic. Such vicious cycles (which scientists call “positive feedbacks” or “runaway feedbacks”) have happened on earth before: it’s believed, for example, that ice ages ended relatively rapidly because of positive feedback cycles in which rising temperatures caused surface snow and ice to melt, which reduced the ground’s reflection of sunlight, which meant the ground absorbed more heat, which led to increased temperatures. (Melted snow – water – is much darker than frozen snow.) Another positive feedback possibility to worry about involves methane hydrates, which are frozen in gigaton quantities in places like Arctic Siberia, and in 100-gigaton quantities on continental shelves. Global warming greater than 1 °C would possibly melt methane hydrates, 7 which release methane into the atmosphere, and methane increases global warming more strongly than CO2 does.
This isn’t the place to discuss the uncertainties of climate change in any more detail. I highly recommend the books Avoiding Dangerous Climate Change (Schellnhuber et al., 2006) and Global Climate Change (Dessler and Parson, 2006). Also the papers by Hansen et al. (2007) and Charney et al. (1979).
The purpose of this chapter is to discuss the idea of fixing climate change by sucking carbon dioxide from thin air; we discuss the energy cost of this sucking next.
The cost of sucking
Today, pumping carbon out of the ground is big bucks. In the future, perhaps pumping carbon into the ground is going to be big bucks. Assuming that inadequate action is taken now to halt global carbon pollution, perhaps a coalition of the willing will in a few decades pay to create a giant vacuum cleaner, and clean up everyone’s mess.
Before we go into details of how to capture carbon from thin air, let’s discuss the unavoidable energy cost of carbon capture. Whatever technologies we use, they have to respect the laws of physics, and unfortunately grabbing CO2 from thin air and concentrating it requires energy. The laws of physics say that the energy required must be at least 0.2 kWh per kg of CO2 (table 31.5). Given that real processes are typically 35% efficient at best, I’d be amazed if the energy cost of carbon capture is ever reduced below 0.55 kWh per kg.
Now, let’s assume that we wish to neutralize a typical European’s CO2 output of 11 tons per year, which is 30 kg per day per person. The energy required, assuming a cost of 0.55 kWh per kg of CO2, is 16.5 kWh per day per person. This is exactly the same as British electricity consumption. So powering the giant vacuum cleaner may require us to double our electricity production – or at least, to somehow obtain extra power equal to our current electricity production.
If the cost of running giant vacuum cleaners can be brought down, brilliant, let’s make them. But no amount of research and development can get round the laws of physics, which say that grabbing CO2 from thin air and concentrating it into liquid CO2 requires at least 0.2 kWh per kg of CO2.
Now, what’s the best way to suck CO2 from thin air? I’ll discuss four technologies for building the giant vacuum cleaner:
- chemical pumps;
- trees;
- accelerated weathering of rocks;
- ocean nourishment.
A. Chemical technologies for carbon capture
The chemical technologies typically deal with carbon dioxide in two steps.
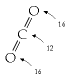
[\text{0.03\%~}\text{CO}{\text{2}}\overset{\text{concentrate}}{\longrightarrow}\text{Pure~}\text{CO}{\text{2}}\overset{\text{compress}}{\longrightarrow}\text{Liquid~}\text{CO}_{\text{2}}]
First, they concentrate CO2 from its low concentration in the atmosphere; then they compress it into a small volume ready for shoving somewhere (either down a hole in the ground or deep in the ocean). 8 Each of these steps has an energy cost. The costs required by the laws of physics are shown in table 31.5.
| cost (kWh/kg) | |
|---|---|
| concentrate | 0.13 |
| compress | 0.07 |
| total | 0.20 |
In 2005, the best published methods for CO2 capture from thin air were quite inefficient: the energy cost was about 3.3 kWh per kg, with a financial cost of about $140 per ton of CO2. 10 At this energy cost, capturing a European’s 30 kg per day would cost 100 kWh per day – almost the same as the European’s energy consumption of 125 kWh per day. Can better vacuum cleaners be designed?
Recently, Wallace Broecker, climate scientist, 11 “perhaps the world’s foremost interpreter of the Earth’s operation as a biological, chemical, and physical system,” has been promoting an as yet unpublished technology developed by physicist Klaus Lackner for capturing CO2 from thin air. Broecker imagines that the world could carry on burning fossil fuels at much the same rate as it does now, and 60 million CO2-scrubbers (each the size of an up-ended shipping container) will vacuum up the CO2. What energy does Lackner’s process require? In June 2007 Lackner told me that his lab was achieving 1.3 kWh per kg, but since then they have developed a new process based on a resin that absorbs CO2 when dry and releases CO2 when moist. Lackner told me in June 2008 that, in a dry climate, the concentration cost has been reduced to about 0.18–0.37 kWh of low-grade heat per kg CO2. The compression cost is 0.11 kWh per kg. Thus Lackner’s total cost is 0.48 kWh or less per kg. For a European’s emissions of 30 kg CO2 per day, we are still talking about a cost of 14 kWh per day, of which 3.3 kWh per day would be electricity, and the rest heat.
Hurray for technical progress! But please don’t think that this is a small cost. We would require roughly a 20% increase in world energy production, just to run the vacuum cleaners.
B. What about trees?

1 hectare = 10 000 m2
Trees are carbon-capturing systems; they suck CO2 out of thin air, and they don’t violate any laws of physics. They are two-in-one machines: they are carbon-capture facilities powered by built-in solar power stations. They capture carbon using energy obtained from sunlight. The fossil fuels that we burn were originally created by this process. So, the suggestion is, how about trying to do the opposite of fossil fuel burning? How about creating wood and burying it in a hole in the ground, while, next door, humanity continues digging up fossil wood and setting fire to it? It’s daft to imagine creating buried wood at the same time as digging up buried wood. Even so, let’s work out the land area required to solve the climate problem with trees.
The best plants in Europe capture carbon at a rate of roughly 10 tons of dry wood per hectare per year 12 – equivalent to about 15 tons of CO2 per hectare per year – so to fix a European’s output of 11 tons of CO2 per year we need 7500 square metres of forest per person. This required area of 7500 square metres per person is twice the area of Britain per person. And then you’d have to find somewhere to permanently store 7.5 tons of wood per person per year! At a density of 500 kg per m3, each person’s wood would occupy 15 m3 per year. A lifetime’s wood – which, remember, must be safely stored away and never burned – would occupy 1000 m3. That’s five times the entire volume of a typical house. If anyone proposes using trees to undo climate change, they need to realise that country-sized facilities are required. I don’t see how it could ever work.
C. Enhanced weathering of rocks
Is there a sneaky way to avoid the significant energy cost of the chemical approach to carbon-sucking? Here is an interesting idea: pulverize rocks that are capable of absorbing CO2, and leave them in the open air. 13 This idea can be pitched as the acceleration of a natural geological process. Let me explain.
Two flows of carbon that I omitted from figure 31.3 are the flow of carbon from rocks into oceans, associated with the natural weathering of rocks, and the natural precipitation of carbon into marine sediments, which eventually turn back into rocks. These flows are relatively small, involving about 0.2 Gt C per year (0.7 Gt CO2 per year). So they are dwarfed by current human carbon emissions, which are about 40 times bigger. But the suggestion of enhanced-weathering advocates is that we could fix climate change by speeding up the rate at which rocks are broken down and absorb CO2. The appropriate rocks to break down include olivines or magnesium silicate minerals, which are widespread. The idea would be to find mines in places surrounded by many square kilometres of land on which crushed rocks could be spread, or perhaps to spread the crushed rocks directly on the oceans. Either way, the rocks would absorb CO2 and turn into carbonates and the resulting carbonates would end up being washed into the oceans. To pulverize the rocks into appropriately small grains for the reaction with CO2 to take place requires only 0.04 kWh per kg of sucked CO2. Hang on, isn’t that smaller than the 0.20 kWh per kg required by the laws of physics? Yes, but nothing is wrong: the rocks themselves are the sources of the missing energy. Silicates have higher energy than carbonates, so the rocks pay the energy cost of sucking the CO2 from thin air.
I like the small energy cost of this scheme but the difficult question is, who would like to volunteer to cover their country with pulverized rock?
D. Ocean nourishment
One problem with chemical methods, tree-growing methods, and rock-pulverizing methods for sucking CO2 from thin air is that all would require a lot of work, and no-one has any incentive to do it – unless an international agreement pays for the cost of carbon capture. At the moment, carbon prices are too low.
A final idea for carbon sucking might sidestep this difficulty. The idea is to persuade the ocean to capture carbon a little faster than normal as a by-product of fish farming. 14
Some regions of the world have food shortages. There are fish shortages in many areas, because of over-fishing during the last 50 years. The idea of ocean nourishment is to fertilize the oceans, supporting the base of the food chain, enabling the oceans to support more plant life and more fish, and incidentally to fix more carbon. Led by Australian scientist Ian Jones, the ocean nourishment engineers would like to pump a nitrogencontaining fertilizer such as urea into appropriate fish-poor parts of the ocean. They claim that 900 km2 of ocean can be nourished to take up about 5 Mt CO2/y. Jones and his colleagues reckon that the ocean nourishment process is suitable for any areas of the ocean deficient in nitrogen. That includes most of the North Atlantic. Let’s put this idea on a map. UK carbon emissions are about 600 Mt CO2/y. So complete neutralization of UK carbon emissions would require 120 such areas in the ocean. The map in figure 31.6 shows these areas to scale alongside the British Isles. As usual, a plan that actually adds up requires country-sized facilities! And we haven’t touched on how we would make all the required urea.
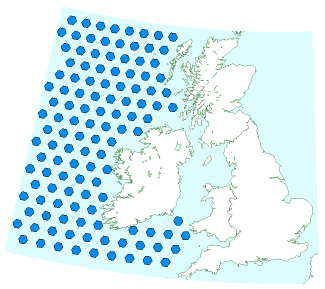

While it’s an untested idea, and currently illegal, I do find ocean nourishment interesting because, in contrast to geological carbon storage, it’s a technology that might be implemented even if the international community doesn’t agree on a high value for cleaning up carbon pollution; fishermen might nourish the oceans purely in order to catch more fish.
Commentators can be predicted to oppose manipulations of the ocean, focusing on the uncertainties rather than on the potential benefits. They will be playing to the public’s fear of the unknown. People are ready to passively accept an escalation of an established practice (e.g., dumping CO2 in the atmosphere) while being wary of innovations that might improve their future well being. They have an uneven aversion to risk.
Ian Jones
We, humanity, cannot release to the atmosphere all, or even most, fossil fuel CO2. To do so would guarantee dramatic climate change, yielding a different planet…
J. Hansen et al (2007)
Avoiding dangerous climate change” is impossible – dangerous climate change is already here. The question is, can we avoid catastrophic climate change?
David King, UK Chief Scientist, 2007
Notes
- climate change… was a controversial question. Indeed there still is a “yawning gap between mainstream opinion on climate change among the educated elites of Europe and America” [voxbz].↩
- Where is the carbon? Sources: Schellnhuber et al. (2006), Davidson and Janssens (2006).↩
- The rate of fossil fuel burning… Source: Marland et al. (2007).↩
- Recent research indicates carbon-uptake by the oceans may be reducing. www.timesonline.co.uk/tol/news/uk/science/ article1805870.ece, www.sciencemag.org/cgi/content/abstract/1136188, [yofchc], Le Quéré et al. (2007).↩
- roughly half of the carbon emissions are staying in the atmosphere. It takes 2.1 billion tons of carbon in the atmosphere (7.5 Gt CO2) to raise the atmospheric CO2 concentration by one part per million (1 ppm). If all the CO2 we pumped into the atmosphere stayed there, the concentration would be rising by more than 3 ppm per year – but it is actually rising at only 1.5 ppm per year.↩
- Radioactive carbon… has penetrated to a depth of only about 400 m. The mean value of the penetration depth of bomb 14C for all observational sites during the late 1970s is 390±39 m (Broecker et al., 1995). From [3e28ed].↩
- Global warming greater than 1 °C would possibly melt methane hydrates. Source: Hansen et al. (2007, p1942).↩
- Shoving the CO2 down a hole in the ground or deep in the ocean. See Williams (2000) for discussion. “For a large fraction of injected CO2 to remain in the ocean, injection must be at great depths. A consensus is developing that the best near-term strategy would be to discharge CO2 at depths of 1000–1500 metres, which can be done with existing technology.” See also the Special Report by the IPCC: www.ipcc.ch/ipccreports/srccs.htm.↩
- In 2005, the best methods for carbon capture were quite inefficient: the energy cost was about 3.3 kWh per kg, with a financial cost of about $140 per ton of CO2. Sources: Keith et al. (2005), Lackner et al. (2001), Herzog (2003), Herzog (2001), David and Herzog (2000).↩
- Wallace Broecker, climate scientist… www.af-info.or.jp/eng/honor/hot/enrbro.html. His book promoting artificial trees: Broecker and Kunzig (2008).↩
- The best plants in Europe capture carbon at a rate of roughly 10 tons of dry wood per hectare per year. Source: Select Committee on Science and Technology.↩
- Enhanced weathering of rocks. See Schuiling and Krijgsman (2006).↩
- Ocean nourishment. See Judd et al. (2008). See also Chisholm et al. (2001). The risks of ocean nourishment are discussed in Jones (2008).↩